Hydrozid®
200 ML
A portable, aerosol cryosurgery device, intended for topical use for the treatment of benign skin lesions. Hydrozid® is intended for treatment of children (5 years and older), adolescents and adults. For professional use only. Medical license required.



Description
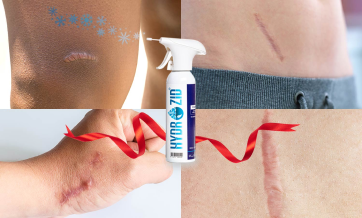
Hydrozid® is used for the treatment of verruca (warts) plantar warts, seborrheic keratosis, actinic keratosis, acrochordon (skin tags), molluscum contagiosum, age spots, dermatofibroma, small keloids, granuloma annulare, porokeratosis plantaris, angiomas, keratoacanthoma, chondrodermatitis, epithelial nevus, leukoplakia, granuloma pyogenicum, pyogenic granuloma, and condylomata acuminata.
The cryogen in Hydrozid® is 1,1,1,2-tetrafluoroethane, more commonly denominated as R134a or norflurane. Norflurane is a nontoxic, nonflammable, odorless, chlorine-free hydrofluroalkane propellant, that does not harm the ozone layer and does not have disposal restrictions. (It can be disposed of in normal household garbage.)
Furthermore, norflurane is well established within the medical industry: For over 60 years it has been used as a propellant in meter-dose asthma inhalers and has proven safe for patients.
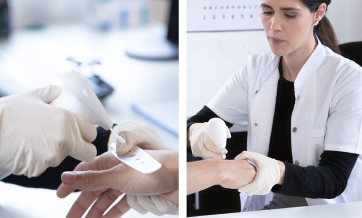
Hydrozid® is sprayed directly to a skin lesion from the canister, utilizing a unique patent-pending spray head. If desired, use the application templates to protect the healthy skin surrounding the target lesion. Each application template includes apertures ranging from 3 to 10 mm. Hydrozid® has a shelf life of 3 years from the time it is manufactured.
Package contents:
- 200 ml Hydrozid® canister of cryogen
- Single-use application templates
- Instructions for Use
Directions
Hydrozid® is used for the treatment of verruca (warts) plantar warts, seborrheic keratosis, actinic keratosis, acrochordon (skin tags), molluscum contagiosum, age spots, dermatofibroma, small keloids, granuloma annulare, porokeratosis plantaris, angiomas, keratoacanthoma, chondrodermatitis, epithelial nevus, leukoplakia, granuloma pyogenicum, pyogenic granuloma, and condylomata acuminata.
The cryogen in Hydrozid® is 1,1,1,2-tetrafluoroethane, more commonly denominated as R134a or norflurane. Norflurane is a nontoxic, nonflammable, odorless, chlorine-free hydrofluroalkane propellant, that does not harm the ozone layer and does not have disposal restrictions. (It can be disposed of in normal household garbage.)
Furthermore, norflurane is well established within the medical industry: For over 60 years it has been used as a propellant in meter-dose asthma inhalers and has proven safe for patients.
Hydrozid® is sprayed directly to a skin lesion from the canister, utilizing a unique patent-pending spray head. If desired, use the application templates to protect the healthy skin surrounding the target lesion. Each application template includes apertures ranging from 3 to 10 mm. Hydrozid® has a shelf life of 3 years from the time it is manufactured.
Package contents:
- 200 ml Hydrozid® canister of cryogen
- Single-use application templates
- Instructions for Use
Safety Information
Pressurized container; may burst if heated. Protect from sunlight.
Do not expose to temperatures exceeding 50°C.
Keep away from the heat, hot surfaces, sparks, open flames and other ignition sources.
Do not pierce or burn canister, even after use.
Contains fluorinated greenhouse gas R134a (0.248 kg 134a; 0.355 t CO2 eq; GWP 1430).
For additional important information see Instructions For Use.