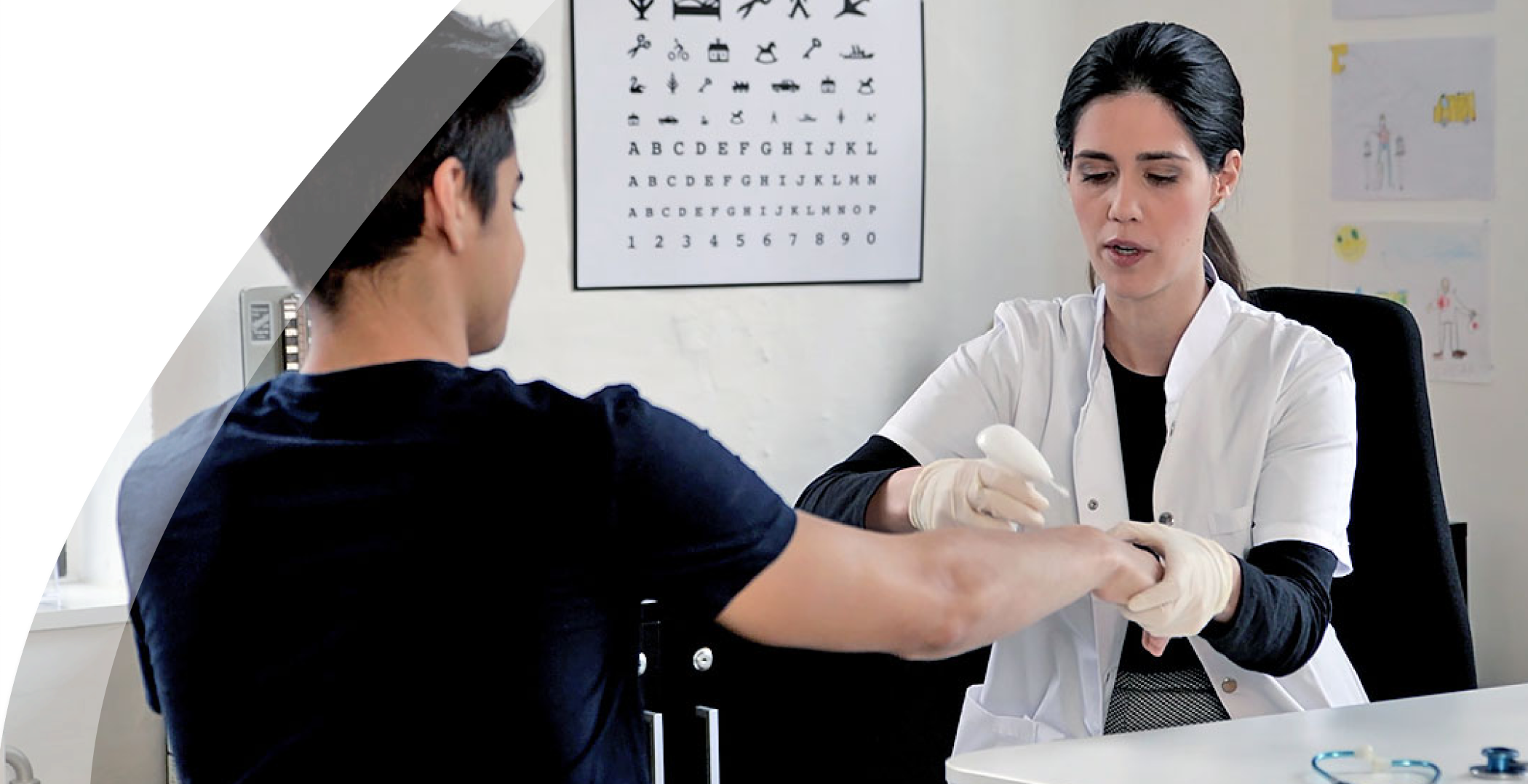
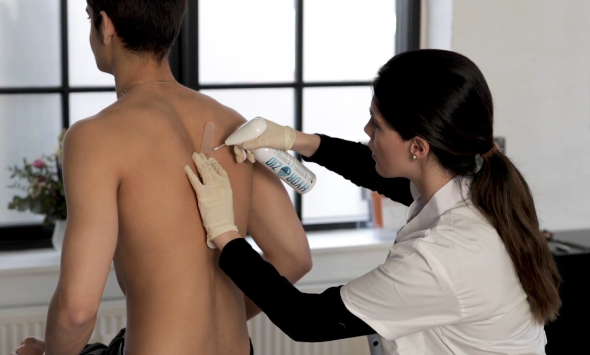
During conversations with Danish doctors and nurses about our novel product, we were met with an abundance of feedback. They expressed a crucial need for a less intimidating alternative to treat warts in children. The sentiment echoed resoundingly: "Imagine if Medilink's product could be harnessed in this manner!"